Transform your vision into reality. Our ExoREADY™ platform empowers you to focus on designing your ideal ocular AAV therapy while we handle the complexities of manufacturing. With streamlined processes, tailored solutions, affordable licensing and transparent communication throughout your journey from concept to clinic.
Unoptimized processes can lead to unexpected delays and increased costs. Our ExoREADY™ platform is pre-optimized to reduce the production timelines to ~9 months (vs compared to the 16-18 months of traditional custom methods).
Small batches, particularly in clinical stages, may be required but not always available with larger CDMOs. We have the flexibility to take on projects of various sizes from 10L to 1000L as you progress your program from preclinical to commercial stages.
Robust QC analytics may not be always available in-house. With our in-house robust QC analytics, you can trust the quality and reliability of your product every step of the way.
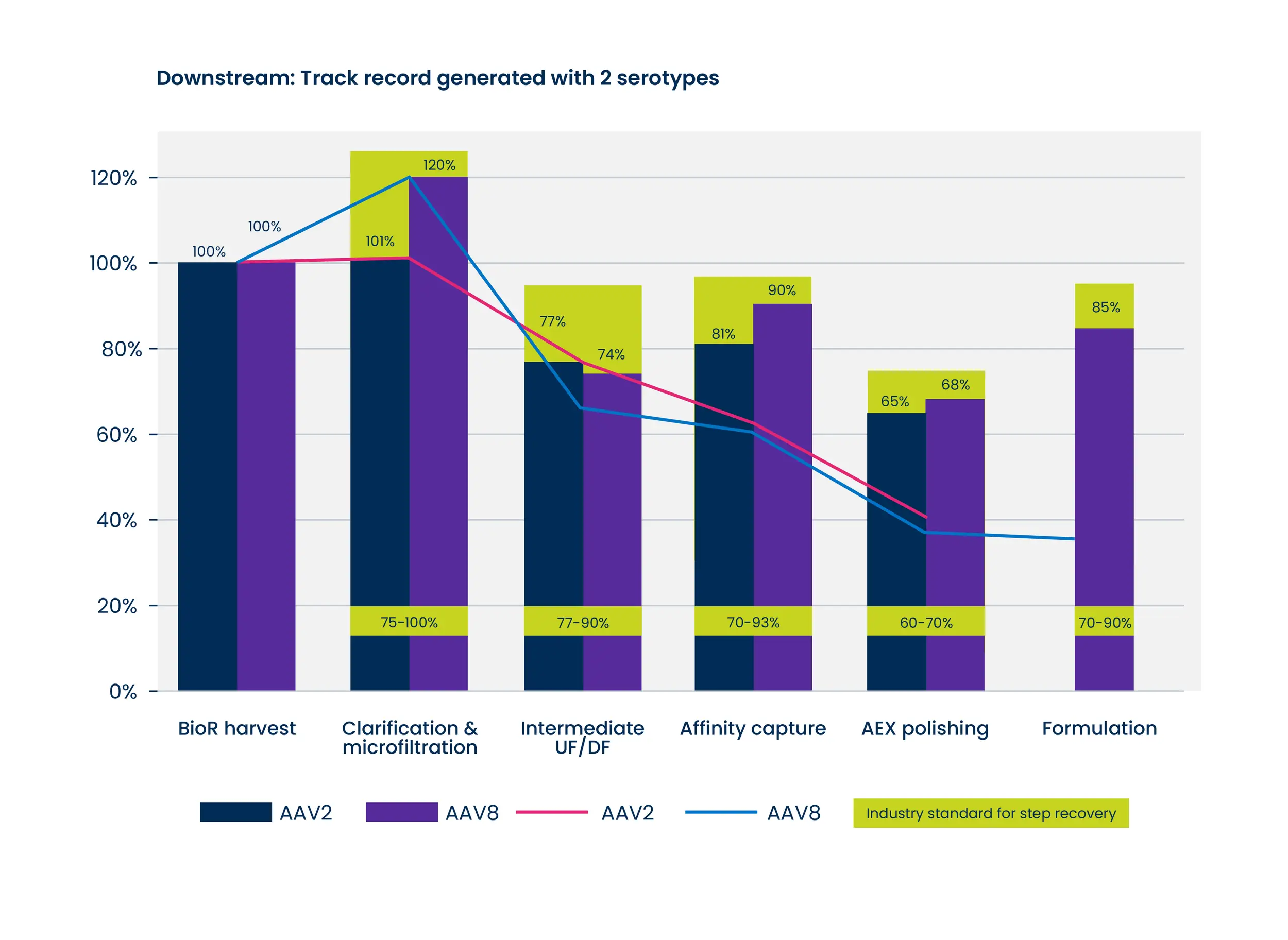
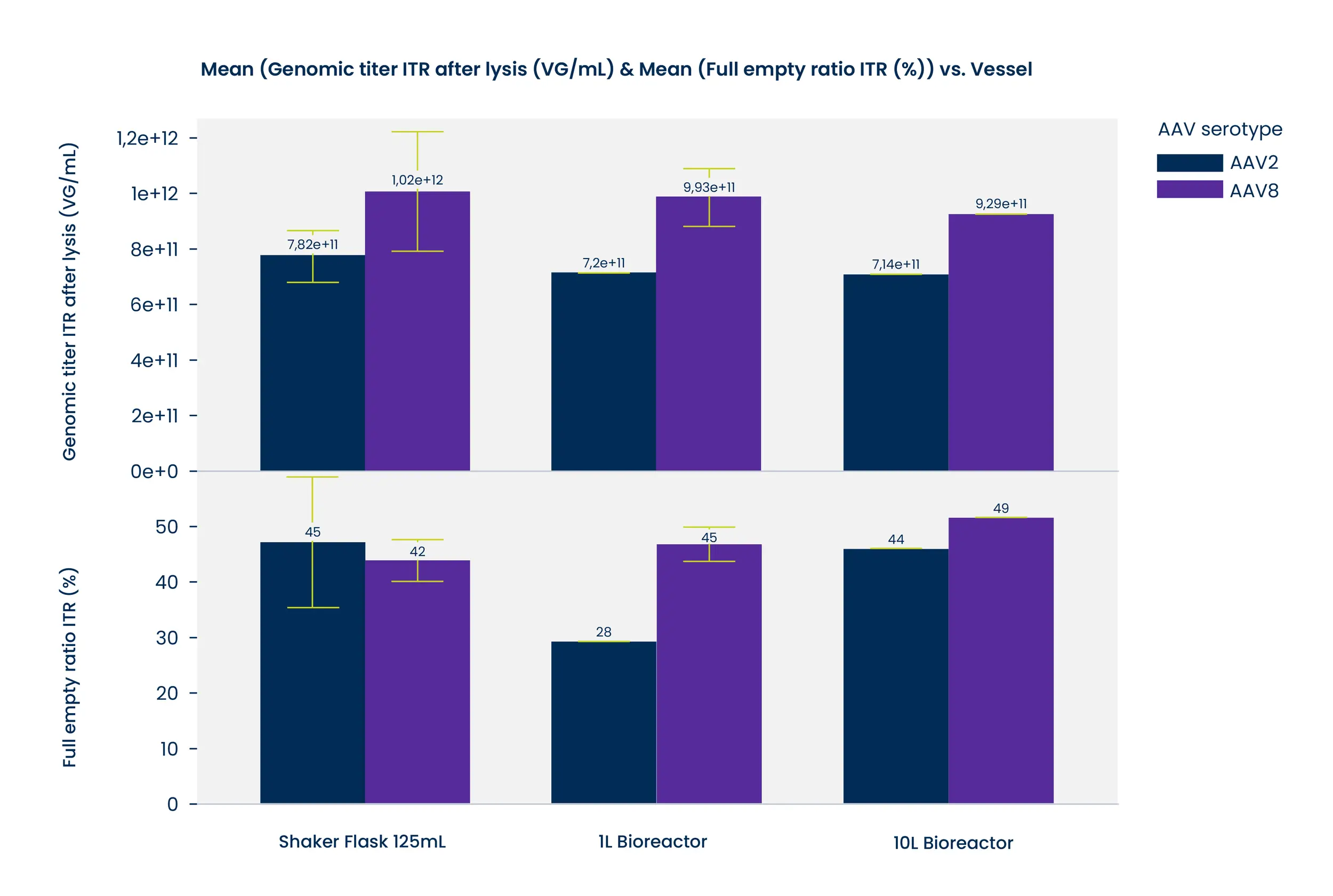
Empty Capsids can result in reduced potency for your drug product. Our optimized ExoREADY™ process delivers consistently high full/empty ratios up to 95% full depending on serotype.
Upholding stringent quality standards with more inexperienced CDMOs can be an issue. We offer CMC consultancy to advise on regulatory aspects of your program.
Excessive licensing fees can result in unsustainable costs for production. The ExoREADY™ platform utilizes in-licensed cell lines and plasmids allow us to avoid licensing fees through phase 3 with competitive licensing rates for later stages.
Unlock the full potential of your research with ExoREADY™. Our advanced platform offers streamlined processes and tailored solutions to fast-track your ocular AAV projects from inception to commercialization.
Experience faster turnaround times and precise results with our in-house analytics. Gain greater control over quality and data integrity, reduce costs, and customize processes to meet your project's needs.
We understand you have specific requirements in terms of cost, recovery, and full/empty ratio. Our flexible platform allows us to tailor solutions to the ensure optimal outcomes.
In Belgium, our state-of-the-art facilities, called VEGA and NOVA, are based on the Univercells Campus, in Jumet (Belgium), close to the Brussels South Airport. They offer 900 m² (9,687 ft²) of GMP production area for viral vectors and 500 m² (5,381 ft²) of ready-to-use production area for nucleic acids.
In North America, our 800m² (8611 ft²). Research Center of Excellence for RUO RNA manufacturing is located in Andover, Massachusetts.
Meet Our Experts
Let's discuss how Exothera's ExoREADY™ platform can empower your next gene therapy breakthrough. Get in touch by completing the form below: